NEWS
Acting Director’s Letter on Sexual Harassment
 The National Institutes of Health (NIH) is committed to ensuring a safe and healthy work environment for everyone. This includes a workplace that is free of sexual harassment and any type of bullying, humiliation, intimidation or discrimination.
The National Institutes of Health (NIH) is committed to ensuring a safe and healthy work environment for everyone. This includes a workplace that is free of sexual harassment and any type of bullying, humiliation, intimidation or discrimination.
Image: Robert H. Carter, M.D., NIAMS Acting Director
Fiscal Year (FY) 2019 Funding Plan
The NIAMS is operating under the FY 2019 Department of Defense and Labor, Health and Human Services, and Education Appropriations Act. The interim funding plan for research and training grants represents the most current information as of July 26, 2019.
NIAMS Statement in Support of Diverse and Inclusive Meetings and Conferences
NIH Director Francis S. Collins, M.D., Ph.D., recently issued a statement that “breaking up the subtle (and sometimes not so subtle) bias that is preventing women and other groups underrepresented in science from achieving their rightful place in scientific leadership must begin at the top.” I fully support this commitment to diversity and inclusion. Moving forward, NIAMS leadership will only accept invitations to speak at scientific conferences and meetings that demonstrate a commitment to diversity and inclusion through representation of key participants, including chairs, speakers and panelists.
NIAMS Extramural Researchers Earn PECASE Awards
 NIAMS grantees John E. Harris, M.D., Ph.D., from the University of Massachusetts Medical School, and Joanne M. Kahlenberg, M.D., Ph.D., from the University of Michigan Medical School, were selected by the White House Office of Science and Technology Policy to be among the NIH-funded researchers to receive the 2019 Presidential Early Career Awards for Scientists and Engineers (PECASE). The PECASE is the highest honor bestowed by the United States government to outstanding scientists and engineers who are beginning their independent research careers and who show exceptional promise for leadership in science and technology.
NIAMS grantees John E. Harris, M.D., Ph.D., from the University of Massachusetts Medical School, and Joanne M. Kahlenberg, M.D., Ph.D., from the University of Michigan Medical School, were selected by the White House Office of Science and Technology Policy to be among the NIH-funded researchers to receive the 2019 Presidential Early Career Awards for Scientists and Engineers (PECASE). The PECASE is the highest honor bestowed by the United States government to outstanding scientists and engineers who are beginning their independent research careers and who show exceptional promise for leadership in science and technology.
Targeting the Microbiome To Treat Malnutrition
 In an investigation funded in part by the NIAMS, researchers assessed the gut health and microbiomial characteristics of malnourished Bangladeshi children, developed three microbiome-friendly formulations and tested them in a month-long clinical trial. Children on one formulation showed significantly improved overall health and intestinal health.
In an investigation funded in part by the NIAMS, researchers assessed the gut health and microbiomial characteristics of malnourished Bangladeshi children, developed three microbiome-friendly formulations and tested them in a month-long clinical trial. Children on one formulation showed significantly improved overall health and intestinal health.
An Itch To Scratch: NIH Scientists Identify Potential New Approach to Chronic Problem
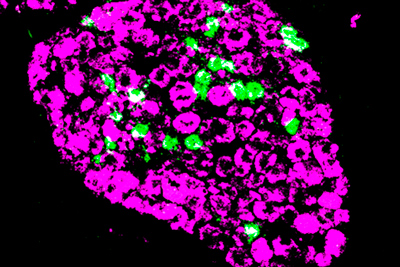 Researchers at the National Center for Advancing Translational Sciences (NCATS) and the National Institute of Dental and Craniofacial Research (NIDCR) have identified candidate compounds to block a neuron receptor associated with itch.
Researchers at the National Center for Advancing Translational Sciences (NCATS) and the National Institute of Dental and Craniofacial Research (NIDCR) have identified candidate compounds to block a neuron receptor associated with itch.
Psoriasis Therapy Linked to Reduced Coronary Inflammation in Patients With the Skin Condition: Novel Imaging Biomarker Could Track Interventions on Coronary Artery Disease
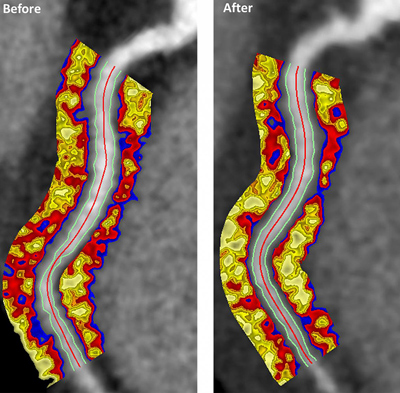 NIH-supported researchers have found that anti-inflammatory biologic therapies used to treat moderate to severe psoriasis can significantly reduce coronary inflammation in patients with the chronic skin condition. The findings involved the use of a novel imaging biomarker that was able to measure the effect of the therapy in reducing the inflammation.
NIH-supported researchers have found that anti-inflammatory biologic therapies used to treat moderate to severe psoriasis can significantly reduce coronary inflammation in patients with the chronic skin condition. The findings involved the use of a novel imaging biomarker that was able to measure the effect of the therapy in reducing the inflammation.
“Wildling” Mice Could Help Translate Results in Animal Models to Results in Humans: NIH Researchers Create Mouse Colony To Address Shortcomings of Laboratory Mice
 NIH researchers, including Markus Hafner, Ph.D., of the NIAMS’ Intramural Laboratory of Muscle Stem Cells and Gene Regulation, have developed a way to make mouse models more reliable in predicting human immune responses.
NIH researchers, including Markus Hafner, Ph.D., of the NIAMS’ Intramural Laboratory of Muscle Stem Cells and Gene Regulation, have developed a way to make mouse models more reliable in predicting human immune responses.
Rare Disease Research Reveals Why Immune Cells Go Wild: Discovery Could Improve Therapy for Multiple Autoimmune Diseases
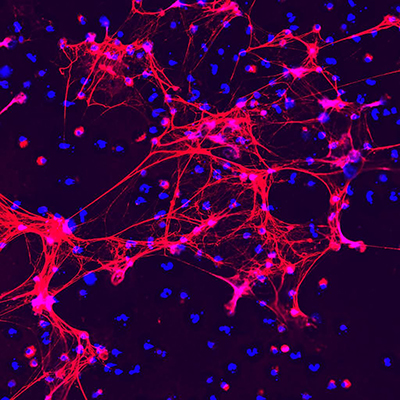 Scientific inspiration can come from unlikely places. A YouTube video spurred Peter Grayson, M.D., M.Sc., of the NIAMS’ Intramural Systemic Autoimmunity Branch, to study a rare disease called DADA2. The research led to new insights that could help improve treatments for patients with a variety of autoimmune disorders.
Scientific inspiration can come from unlikely places. A YouTube video spurred Peter Grayson, M.D., M.Sc., of the NIAMS’ Intramural Systemic Autoimmunity Branch, to study a rare disease called DADA2. The research led to new insights that could help improve treatments for patients with a variety of autoimmune disorders.
FDA Approves First Generics of Lyrica
The U.S. Food and Drug Administration (FDA) has approved multiple applications for generics of Lyrica (pregabalin) for the management of neuropathic pain associated with diabetic peripheral neuropathy, for the management of postherpetic neuralgia, as an adjunctive therapy for the treatment of partial onset seizures in patients 17 years of age and older, for the management of fibromyalgia and for the management of neuropathic pain associated with spinal cord injury.
Other News From FDA
- FDA Clears New Indications for Existing Lyme Disease Tests That May Help Streamline Diagnoses
- FDA Approves Boxed Warning About Increased Risk of Blood Clots and Death With Higher Dose of Arthritis and Ulcerative Colitis Medicine Tofacitinib (Xeljanz, Xeljanz XR)
- FDA Approves First of Its Kind Device To Treat Pediatric Patients With Progressive Idiopathic Scoliosis
RESOURCES
Spotlight on Scientific Imagery: Bone Growth Near a Fracture
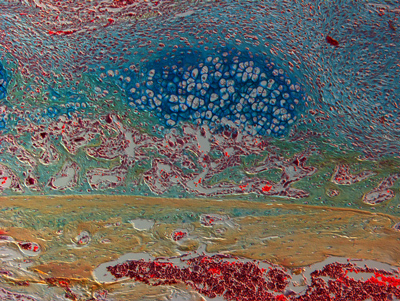 This image shows a section of mouse bone near a fracture, with marrow full of red blood cells at the bottom and healthy bone on top of that (tan). New bone (green) and cartilage (dark blue) are in the upper layer. Researchers suggest that chronic inflammation is the reason that bones do not heal as well as we age, as opposed to simply the passage of time.
This image shows a section of mouse bone near a fracture, with marrow full of red blood cells at the bottom and healthy bone on top of that (tan). New bone (green) and cartilage (dark blue) are in the upper layer. Researchers suggest that chronic inflammation is the reason that bones do not heal as well as we age, as opposed to simply the passage of time.
Photo credit: Philipp Leucht, M.D., Ph.D., New York University School of Medicine
NIH News in Health: What Is Psoriatic Arthritis?
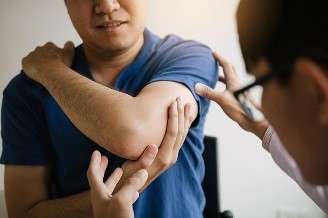 Psoriasis is an autoimmune disease that causes scaly patches to appear on the skin. People who get psoriasis may also get psoriatic arthritis, which causes swelling and pain in the joints. Your health care provider can help you find out if you have psoriatic arthritis and recommend treatment methods that are best for you.
Psoriasis is an autoimmune disease that causes scaly patches to appear on the skin. People who get psoriasis may also get psoriatic arthritis, which causes swelling and pain in the joints. Your health care provider can help you find out if you have psoriatic arthritis and recommend treatment methods that are best for you.
New Video About NIH
 This video captures the spirit of the NIH, the NIH-funded community and all the people who make it possible.
This video captures the spirit of the NIH, the NIH-funded community and all the people who make it possible.
EVENTS
September NIAMS Advisory Council Meeting
The next NIAMS Advisory Council Meeting will be held September 10, 2019 on the NIH campus in Building 45, Rooms E1/E2. The Council meeting will be available for live viewing via the NIH videocasting service as well.

National Center for Complementary and Integrative Health (NCCIH) 20th Anniversary Research Symposium
September 23, 2019
9 a.m. to 4:30 p.m.
Lipsett Amphitheater, Building 10, NIH Campus, Bethesda, Maryland
The event will be videocast.
FDA Public Workshop: Accelerating Drug Development for Polyarticular Juvenile Idiopathic Arthritis (pJIA) 
October 2, 2019
8 a.m. to 5 p.m.
White Oak Campus, 10903 New Hampshire Avenue, Building 31, Silver Spring, Maryland
Registration ![]() is required for in-person or webcast attendance.
is required for in-person or webcast attendance.
NIH Director's Wednesday Afternoon Lecture Series
The NIH Director’s Wednesday Afternoon Lecture Series resumes in September. Renowned scientists from around the globe present research weekly on a variety of topics. The lectures are continuing medical education certified, open to the public and available live via webcast.
NIH Science Lectures and Events Available via Internet
The NIH hosts a number of science seminars and events that are available online through real-time streaming video (videocast). The NIH calendar notes these videocast events with a video icon ![]() .
.
