NEWS
Fiscal Year (FY) 2020 Funding Plan
The NIAMS is operating under the FY 2020 Further Consolidated Appropriations Act. The interim funding plan for research and training grants represents the most current information as of May 29, 2020.
COVID-19 Updates
COVID-19 is an emerging, rapidly evolving situation. Get the latest public health information from the Centers for Disease Control and Prevention (CDC), and the latest research information from the National Institutes of Health (NIH). Additional resources include:
- NIAMS Jumpstarts Volunteer Response to Coronavirus
- COVID-19: Information for NIH Applicants and Recipients of NIH Funding from the NIH Office of Extramural Research
- National Institute of Allergy and Infectious Diseases (NIAID): Coronaviruses
- Coronavirus (COVID-19) Update: U.S. Food and Drug Administration (FDA) Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine
Piecing Together the Puzzle of Chronic Low Back Pain: A Computer Model May Be Able To Inspire New Insights and Treatments
To address the problem of chronic low back pain, a team of researchers funded by the Helping to End Addiction Long-termSM (HEAL) Initiative, specifically the Back Pain Consortium (BACPAC) Research Program, is creating a whole-system model to represent everything that contributes to chronic low back pain or helps treat it – from anxiety to tissue damage and from psychotherapy to surgery.
NIH HEAL Initiative’s BACPAC Program Video: Understanding Back Pain
NIAMS Acting Director, Robert H. Carter, M.D., discusses the BACPAC Research Program, part of the NIH HEAL InitiativeSM: “The ultimate goal of the Back Pain Consortium is not just to understand what causes the pain, but to understand it in each individual.”
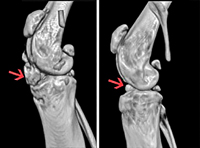
Blocking Enzyme Inhibits Arthritis Progression in Mice
Researchers funded by the NIAMS and the National Science Foundation demonstrated that the loss of TET1, an enzyme that affects gene changes associated with osteoarthritis (OA), protected mice against the development of OA. In addition, blocking TET1 in cells taken from people with osteoarthritis also prevented gene changes that help drive the disease. These results suggest a potential strategy for arthritis drug development.
Hairy Human Skin Generated From Stem Cells
Scientists created complex hair-growing skin from human stem cells and successfully grafted it to mice in a project supported in part by the NIAMS. The lab-grown skin may help advance research into skin development and diseases. While challenges remain, it could have implications for skin reconstruction after burns and wounds.
Targeted Approach for Tackling Sjögren’s Syndrome
Using chemical compounds that target cell signaling, researchers from the National Institute of Dental and Craniofacial Research (NIDCR) and NIH-supported colleagues restored salivation in mouse models of Sjögren’s syndrome, a disorder in which the body’s immune response impairs salivary gland function. The results suggest that this approach could be explored for its therapeutic potential in some people with Sjögren’s syndrome.
FDA Approves First Treatment for Adult Onset Still’s Disease, a Severe and Rare Disease
The FDA approved Ilaris (canakinumab) injection for the treatment of active Still’s disease, including adult-onset Still’s disease. Ilaris was previously approved for systemic juvenile idiopathic arthritis in patients ages 2 years and older.
Funding Opportunities
Rapid Acceleration of Diagnostics (RADx) Funding Opportunity Announcements
The NIH’s RADx is an initiative that aims to speed development, delivery and use of COVID-19 testing technologies. The NIH has issued four new Funding Opportunity Announcements for the RADx Underserved Populations program, seeking research applications specifically for expanding access to and acceptance of testing in underserved and/or vulnerable populations that have been disproportionately affected by the pandemic. Applications are due on August 7, 2020, or September 8, 2020, depending on the announcement.
NIAMS Encourages Applications for the NIAMS Resource-based Centers Program (P30) for Research Areas Within its Mission in Bone, Muscle and Orthopaedic Research
The NIAMS has issued a Request for Applications (RFA-AR-21-004) for P30 Centers for critical research infrastructure, shared facilities, services and/or resources to groups of investigators conducting research on bone, muscle and orthopaedic biology and diseases. The broad overall goal is to accelerate, enrich and enhance the effectiveness of ongoing basic, translational and clinical research and to promote new research within these areas of the NIAMS mission. The deadline for applications is August 11, 2020.
NIAMS Encourages Applications for the Regenerative Medicine Innovation Project (RMIP) Investigator-Initiated Clinical Trials (UG3/UH3 – Clinical Trial Required)
The NIAMS is a participating Institute in the NIH Request for Applications (RFA-HL-21-003) for highly meritorious clinical trial applications proposing to explore and enable the development of safe and effective regenerative medicine (RM) interventions using adult stem cells. The deadline for applications is October 2, 2020.
NIAMS Encourages Applications for the NIAMS Resource-based Centers Program (P30) for Research Areas Within its Mission in Rheumatic Research
The NIAMS has issued a Request for Applications (RFA-AR-21-002) for P30 Centers for critical research infrastructure, shared facilities, services and/or resources to groups of investigators conducting research on rheumatic diseases. The broad overall goal is to accelerate, enrich and enhance the effectiveness of ongoing basic, translational and clinical research and to promote new research on rheumatic diseases within the NIAMS mission. The deadline for applications is October 6, 2020.
NIAMS Encourages Request for Information: Challenges and Opportunities in Improving Our Understanding of Transposable Elements and Somatic Mosaicism
The NIAMS is a participating Institute in the NIH Request for Information (NOT-RM-20-020), as the agency is considering the possibility of developing a Common Fund program to better understand the relationship between transposable elements, somatic mosaicism and human tissue function. The NIH is seeking input in order to help identify the needs and priorities in this area of science, and to plan future activities and initiatives that can most significantly impact biomedical research. The deadline for responses is July 17, 2020.
FDA Requests Input on Rare Disease Clinical Trial Networks
As part of the FDA Rare Disease Cures Accelerator Program, the FDA announced a Request for Information and Comment on Rare Disease Clinical Trial Networks to get public input – including from patients, patient advocates, the scientific community and health professionals – on practical steps and successful approaches relating to the start-up, implementation and sustainment of global clinical trials networks, including specific considerations for establishing such networks for a range of rare diseases. Comments are due by July 31, 2020.
Stay Updated About Funding Announcements
If you would like information about funding opportunities, please view the NIH Guide for Grants and Contracts, the primary source for information about NIH funding opportunities. You can also request a weekly Table of Contents from the NIH Guide. In addition, the NIAMS website provides comprehensive information on NIAMS-related grants and processes.
RESOURCES
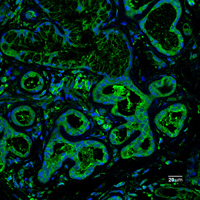
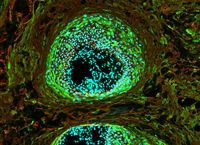
Spotlight on Scientific Imagery: Keratin Skin Tumors
This image shows Keratin 5 (red) and Keratin 1 (green) expression in skin tumors. Keratins are fibrous structural proteins present in epithelial structures, such as skin, hair and nails. They form filaments that give strength and toughness to the structures. Keratins are often used to assist in the diagnosis of squamous cell carcinoma, a form of skin cancer.
Photo credit: Elisabetta Palazzo, Ph.D., NIAMS Laboratory of Skin Biology
CDC Releases Arthritis Resources, Including a New Report: A National Public Health Agenda for Osteoarthritis
The CDC, in partnership with the Arthritis Foundation and the OA Action Alliance, released A National Public Health Agenda for Osteoarthritis (2020) [PDF – 330MB]. The agenda lays out strategies and goals through which stakeholders can improve the health and quality of life of millions of Americans with OA. Other new resources include:
- The Arthritis-Mental Health Connection
- Managing Arthritis in Rural Areas
- Physical Activity Helps Arthritis Pain
EVENTS
June NIAMS Advisory Council Meeting Available on Videocast
A video recording of the June 9 NIAMS Advisory Council Meeting is available. The next NIAMS Advisory Council Meeting will be held virtually on September 1, 2020.
NIH Science Lectures and Events Available via Internet
Premature Cardiovascular Disease in Systemic Autoimmunity: Lessons Learned From Lupus
Recorded Wednesday, June 17, 2020
Mariana J. Kaplan, M.D., Senior Investigator and Chief, Systemic Autoimmunity Branch, NIAMS; and Deputy Scientific Director, NIAMS, NIH
Lighting Up Our Lives: How Light Influences Our Mental and Physical Health: Novel Approaches at the Intersection of Mental Health and Pain
Helen Burgess, Ph.D., University of Michigan
June 30, 2020
2020 National Institute of Nursing Research (NINR) Artificial Intelligence Boot Camp
August 3–6, 2020
Registration opens July 1.
For additional online science seminars and events hosted by the NIH, view the NIH calendar, which notes videocast events with a video icon ![]() .
.
Funding Announcements
If you would like information about funding opportunities, please view the NIH Guide to Grants and Contracts, the primary source for information about NIH funding opportunities. You can also request a weekly Table of Contents from the NIH Guide. In addition, the NIAMS website provides comprehensive information on NIAMS-related grants and processes.